Recent Articles
Home » Archives for February 2015
Wednesday 18 February 2015
Wednesday 18 February 2015
- 0 Comments
Ebola Virus Pathogenesis: Implications for Vaccines and Therapies
Ebola virus is an aggressive pathogen that causes a highly lethal hemorrhagic fever syndrome in humans and nonhuman primates. First recognized near the Ebola River valley during an outbreak in Zaire in 1976 (6, 20), outbreaks have occurred in Africa in the ensuing 27 years, with mortality rates ranging from 50 to 90% (26, 28). Outbreaks have been identified yearly for the past 3 years in central Africa, the most recent of which continues in the Republic of the Congo, with more than 125 fatalities to date according to the World Health Organization (http://www.who.int/csr/don/2003_05_07/en/, accessed 7 May 2003). The natural host for Ebola virus is unknown, so it has not been possible to implement programs to control or eliminate viral reservoirs of transmission to human populations. The rapid progression of Ebola virus infection has further complicated the control of this disease, affording little opportunity to develop acquired immunity. There is currently no antiviral therapy or vaccine that is effective against Ebola virus infection in humans.
Although its clinical course is well known, the specific mechanisms underlying the pathogenicity of Ebola virus have not been clearly delineated. This is due, in part, to the difficulty in obtaining samples and studying the disease in the relatively remote areas in which the outbreaks occur. In addition, a high degree of biohazard containment is required for laboratory studies and clinical analysis. Isolation of the viral cDNAs and the development of expression systems have allowed the study of Ebola virus gene products under less restrictive conditions and facilitated an understanding of the mechanisms underlying virally induced cell damage.
EBOLA VIRUS DISEASE PROGRESSION
Typically, Ebola virus infection runs its course within 14 to 21 days. Infection initially presents with nonspecific flu-like symptoms such as fever, myalgia, and malaise. As the infection progresses, patients exhibit severe bleeding and coagulation abnormalities, including gastrointestinal bleeding, rash, and a range of hematological irregularities, such as lymphopenia and neutrophilia. Cytokines are released when reticuloendothelial cells encounter virus, which can contribute to exaggerated inflammatory responses that are not protective. Damage to the liver, combined with massive viremia, leads to disseminated intravascular coagulopathy. The virus eventually infects microvascular endothelial cells and compromises vascular integrity. The terminal stages of Ebola virus infection usually include diffuse bleeding, and hypotensive shock accounts for many Ebola virus fatalities (for reviews, see references 9 and 28).
STRUCTURE AND CLASSIFICATION OF THE EBOLA VIRUS
Ebola virus and the related Marburg virus are members of the Filovirus family, which are pleomorphic, negative-sense RNA viruses whose genome organization is most similar to the Paramyxoviridae. Of the four identified strains of Ebola virus, three—the Zaire, Ivory Coast, and Sudan strains—have been shown to cause disease in both humans and nonhuman primates, with the Zaire strain exhibiting the highest lethality rate (13, 29). The only documented outbreaks of Ebola virus infection in the United States were fortunately restricted to nonhuman primates at holding facilities in Virginia and Texas, caused by the Reston strain, which has not yet caused fatal disease in humans (19).
The Ebola virus genome is 19 kb long, with seven open reading frames encoding structural proteins, including the virion envelope glycoprotein (GP), nucleoprotein (NP), and matrix proteins VP24 and VP40; nonstructural proteins, including VP30 and VP35; and the viral polymerase (reviewed in reference 28). Unlike that of Marburg virus, the GP open reading frame of Ebola virus gives rise to two gene products, a soluble 60- to 70-kDa protein (sGP) and a full-length 150- to 170-kDa protein (GP) that inserts into the viral membrane (29, 41), through transcriptional editing.
EBOLA VIRUS GP AND VIRAL PATHOGENESIS
The Ebola virus GP is synthesized in a secreted (sGP) or full-length transmembrane form, and each gene product has distinct biochemical and biological properties. For example, GP appears to form a trimeric complex (30) and binds preferentially to endothelial cells, whereas sGP does not (49). Preferential binding of Ebola virus GP to the endothelium was demonstrated by use of two independent methodologies as follows: direct binding was assessed by fluorescence-activated cell sorter analysis, and pseudotyping experiments were performed in which virus titers, cell numbers, and confluence were carefully determined so that the multiplicity of infection was controlled and equal in all cell types. Another study failed to demonstrate this preferential binding (17), but direct binding of GP to endothelial cells was not measured and neither the multiplicity of infection, target cell numbers, nor cell confluence was reported in that study. The receptors required for cell binding and infection are not completely understood. A folate-related receptor can serve as a cofactor to facilitate infection (8), but whether it serves as a receptor remains unclear. The cell surface lectin DC-SIGN can also facilitate GP binding to cells through viral carbohydrate determinants, but it does not appear to mediate entry by itself (1, 32). In contrast to GP, sGP gives rise to a dimeric protein (30) that interacts with neutrophils (49). sGP mediates neutrophil binding, directly or indirectly, through CD16b, the neutrophil-specific form of the Fcγ receptor III (49). After the initial description of the neutrophil binding of sGP, it was shown that immunoglobulin G (IgG), but not an Fab fragment, against sGP was needed to detect neutrophil binding (T. Maruyama, M. J. Buchmeier, P. W. H. I. Parren, and D. R. Burton, Technical Comment, Science 282:843-844, 1998). A subsequent study showed that the binding could also be seen if an irrelevant IgG was used with the Fab fragment against sGP (Z.-Y. Yang, R. Delgado, L. Xu, R. F. Todd, E. G. Nabel, A. Sanchez, and G. J. Nabel, Author's Reply, Science 282:844-846, 1998). Though such binding could potentially arise from binding of immune complexes, additional studies using resonance energy transfer showed that neutrophils incubated with sGP showed a significant reduction in the CR3-Fcγ RIIIB RET signal (22), demonstrating that sGP alters the physical and functional interaction between Fcγ RIIIB and CR3. Through this interaction, sGP may contribute to immune evasion by inhibiting early steps in neutrophil activation (as measured by the down-modulation of l-selectin) that would ordinarily assist in virus clearance (49).
Several lines of evidence suggest that the viral GP plays a key role in the manifestations of Ebola virus infection. The transmembrane form of GP targets the Ebola virus to cells that are relevant to its pathogenesis. Specifically, GP allows the virus to introduce its contents into monocytes and/or macrophages, where cell damage or exposure to viral particles may cause the release of cytokines (34) associated with inflammation and fever, and into endothelial cells, which damages vascular integrity (48) (Fig. (Fig.1).1). Thus, sGP may alter the immune response by inhibiting neutrophil activation, while the transmembrane GP may contribute to the hemorrhagic fever symptoms by targeting virus to cells of the reticuloendothelial network and the lining of blood vessels.

Host immune responses to Ebola virus and cell damage due to direct infection of monocytes and macrophages cause the release of cytokines associated with inflammation and fever (A). Infection of endothelial cells also induces a cytopathic effect and damage ...
GP expression in cultured human endothelial and epithelial cells causes cell rounding and detachment (48). GP is the only one of the seven Ebola virus gene products to exert this effect, and though GP from all four documented Ebola virus strains acts similarly, the highly pathogenic Zaire strain has the most potent activity in this cell culture assay (33). These effects require the presence of the mucin-like, serine-and-threonine-rich domain of GP and correspond with the down-regulation of specific molecules on the cell surface (48). Cytotoxicity appears to be precisely controlled by a mechanism involving down-regulation of GP expression through a transcriptional RNA editing event by the viral polymerase. The importance of this phenomenon was shown by use of a reverse genetics system for replicating Ebola virus in which a mutation that increases the level of full-length GP expression is significantly more cytotoxic than the wild-type virus (42).
The in vivo relevance of GP-induced endothelial cell toxicity was explored in blood vessel explants (48) in which human saphenous veins were infected with replication-defective adenoviral vectors carrying the gene for GP or sGP. Staining with horseradish peroxidase and scanning electron microscopy were used to observe severe damage to the endothelial cell lining in vessels that received the virus encoding full-length Ebola virus GP but not sGP or vectors in which the mucin domain of GP was removed. Cell damage in explant cultures paralleled the species specificity of different Ebola virus strains: no toxicity was observed when Reston strain GP was introduced into human vascular explants, whereas significant tissue damage was observed in vascular explants from nonhuman primates.
Further in vitro analyses have begun to elucidate the molecular mechanisms underlying GP-induced cytotoxicity. Critical mediators of cell adhesion to the matrix and immune signaling (e.g., integrins and major histocompatibility complex class I cell surface proteins) are among the cell surface molecules that are dysregulated (33, 37). Transient expression of Ebola virus GP in human kidney 293T cells caused a reduction of specific integrins (primary molecules responsible for cell adhesion to the extracellular matrix) on the cell surface. GP mutants lacking the membrane-spanning region of the ectodomain did not cause this down-regulation, suggesting that anchorage of GP to the cell membrane is required for this effect. Disruption of major histocompatibility complex class I expression on the cell surface is a mechanism for evading host immune responses that is shared by several pathogens, including cytomegalovirus, human immunodeficiency virus (HIV), and herpesviruses (27). It is not known whether GP affects integrin levels by altering intracellular trafficking or by modulation of protein synthesis or degradation, but preliminary experiments suggest a role for cellular protein transport machinery in GP-mediated cytotoxicity (N. Sullivan, unpublished observations). In any event, the biologic effects of GP alone may account largely for the features of Ebola virus infection that lead to fatal disease, including inflammatory dysregulation, immune suppression, and loss of vascular integrity.
Structural analyses of GP have revealed features in common with other viral envelope proteins. The crystal structure of the GP ectodomain revealed a coiled-coil domain resembling a trimer of helical hairpin-like loops (23, 44). The hairpin structure is adjacent to the fusion-peptide region (16) hypothesized to insert directly into the target cell membrane. Analogous coiled-coil regions have been defined for GPs of influenza virus, murine retroviruses, HIV, and simian immunodeficiency virus (SIV) as well as for some cellular proteins, called SNARES, that function in intracellular vesicle fusion (44). For HIV gp160, it has been possible to identify peptides that bind to a transient intermediate form that precedes hairpin formation. Because of their potent inhibition of viral entry, these reagents have shown considerable promise in clinical trials (21). The Ebola virus GP contains a homologous hairpin structure for which a possible inhibitory peptide has been identified (43), a region that remains a potential therapeutic target.
IMMUNE RESPONSE TO EBOLA VIRUS INFECTION
Ebola virus replicates at an unusually high rate that overwhelms the protein synthesis apparatus of infected cells and host immune defenses (28). Both the adaptive immune and inflammatory systems respond to infection at the same time that some cell types, specifically monocytes and macrophages, are targets relevant to disease pathogenesis. This feature of the infection was initially suggested by the immunohistochemical localization of Ebola virus in vivo: endothelial cells, mononuclear phagocytes, and hepatocytes are the main targets of infection (3, 4, 12, 50).
The components of the immune system that may protect against Ebola virus infection have not been defined. Antibody titers against Ebola virus GPs are readily detectable in patients who recover from Ebola virus infection; however, anecdotal reports have indicated that serum from recovered patients did not consistently protect against infection or exhibit neutralization of virus replication in cell culture. Furthermore, passive transfer of antibodies in animal models only delays the onset of symptoms and does not alter overall survival (18). More recently, the neutralization of virus replication by selected monoclonal antibodies isolated from the bone marrow of recovered patients was demonstrated in vitro (24), and monoclonal antibodies that recognize specific epitopes of Ebola virus GP have been shown to confer immune protection in a murine model of Ebola virus infection (15,45) and in guinea pigs (25). However, it is relatively easy to protect against infection in the mouse model, and protection of guinea pigs required a high dose of antibody administered very close to the time of virus challenge. Taken together, these results suggest that antibodies alone do not provide protective immunity in a natural context and that cellular immunity is likely to play a significant role in virus clearance. Whether hyperimmune serum from surviving vaccinated animals or certain infrequently occurring antibodies are capable of attenuating infection remains unknown, but such antibodies could potentially contribute to therapy if they can be identified and optimized.
A comparison of immune parameters in survivors and nonsurvivors of infection has provided clues into the constituents of an effective immune response. Baize et al. (2) characterized the immune responses of patients in two large Ebola virus outbreaks in Gabon in 1996. There was no significant difference in viral antigen load between survivors and nonsurvivors, but immune responses varied, suggesting that survival is dependent on the initial or innate immune response to infection. Survivors exhibited more significant IgM responses, clearance of viral antigen, and sustained T-cell cytokine responses, as indicated by high levels of T-cell-related mRNA in the peripheral blood. In contrast, antibodies specific for the virus were nearly undetectable in fatal cases, and while gamma interferon (IFN-γ) was detected early after infection, T-cell cytokine RNA levels were more indicative of a failure to develop adaptive immunity in the days preceding death.
During infection, there is evidence that both host and viral proteins contribute to the pathogenesis of Ebola virus. Increases in the levels of inflammatory cytokines IFN-γ, IFN-α, interleukin-2 (IL-2), IL-10, and tumor necrosis factor alpha were associated with fatality from Ebola hemorrhagic fever (40). Moreover, in vitro experiments demonstrated that tumor necrosis factor released from filovirus-infected monocytes and macrophages increased the permeability of cultured human endothelial cell monolayers (12). However, other reports have observed an association between elevated levels of IFN-γ mRNA and protection from infection (2), and a protective effect of IFN-α and -γ is suggested by the fact that the virus has evolved at least one protein, VP35, that acts as an IFN-α/β antagonist (5). Whether the effects of cytokines are protective or damaging may depend not only on the cytokine profile but also may represent a delicate balance influenced by the route and titer of incoming virus as well as factors specific to the individual host immune response.
VACCINE DEVELOPMENT
Several animal models have been developed to study the pathogenesis of Ebola virus infection and to assess the efficacy of various vaccine approaches. Guinea pigs and nonhuman primates represent the primary animal models for vaccine development because the progression and pathogenesis most closely resemble those of the human disease (10,46, 47). A murine model was later developed by serial passage of virus in mice (7). Though the model allows the use of knockout and inbred strains to evaluate genetic determinants of disease, it is considered less predictive of human disease because it relies on a serially passaged, attenuated virus. While symptoms and time course of disease in guinea pigs parallel those in humans, nonhuman primate infection is considered the most predictive and useful for vaccine development (14).
Live attenuated viruses and recombinant proteins have been used successfully in a variety of vaccines, but the safety and immunogenicity of gene-based vaccines have proven increasingly attractive. Among the gene-based approaches, naked plasmid DNA has been used successfully in animal models to direct the synthesis of immunogens within the host cells and has proven helpful in a variety of infectious diseases (reviewed in references 11and 38).
Genetic immunization with plasmid DNA was developed in the guinea pig and was the first successful vaccine for Ebola virus (47). In this model, NP elicited a primarily humoral response and was less efficacious, while sGP and GP elicited T-cell proliferative and cytotoxic responses as well as a humoral response. Protection against lethal challenge was conferred by each of these immunogens when animals were infected within 1 month of the last immunization, but only GP or sGP provided long-lasting protection. The degree of protection correlated with antibody titer and antigen-specific T-cell responses. Subsequent studies of NP and GP plasmids conferred protective immunity in mice (39), but it is uncertain whether the attenuated murine virus is more sensitive to neutralization than the wild-type virus. Thus, the relative potency of NP, or its requirement as an immunogen for providing long-term protection, remains uncertain.
While DNA vaccines have been highly effective in rodents, their efficacy in nonhuman primates or humans has been less impressive. Priming-boosting immunization protocols that use DNA immunization followed by boosting with poxvirus vectors carrying the genes for pathogen proteins have yielded dramatically enhanced immune responses in animal studies, with 30-fold or greater increases in antibody titer from the booster (31). A different priming-boosting strategy using replication-defective adenovirus for an Ebola virus vaccine was tested in cynomolgus macaques (36). This study demonstrated the superior immunologic efficacy of this priming-boosting combination for both cellular and humoral responses. These animals displayed complete immune protection against a lethal challenge of virus, providing the first demonstration of an Ebola virus vaccine approach that protects primates against infection. Recently, an accelerated vaccination has been developed that confers protection against a lethal virus challenge in nonhuman primates after a single immunization (36a). If this vaccine works similarly in humans, it may be useful in the containment of acute outbreaks by ring vaccination.
In summary, an understanding of the mechanisms underlying Ebola virus-induced cytopathic effects has facilitated the process of vaccine and antiviral therapy development, which has in turn provided new information about pathogenesis and the immune response. Ebola virus does not exhibit the high degree of variability that other enveloped viruses may employ to evade host immunity, but Ebola virus GP alters target-cell function and exemplifies a novel strategy for immune evasion that may have arisen through the evolution of Ebola virus with its natural host. The cytotoxic effects of GP on macrophage and endothelial cell function disrupt inflammatory cell function and the integrity of the vasculature. In addition, by altering the cell surface expression of adhesion proteins and immune recognition molecules, Ebola virus may disrupt processes critical to immune activation and cytolytic-T-cell function. These phenomena likely account for the dysregulation of the inflammatory response and the vascular dysfunction characteristic of lethal Ebola virus infection, providing a rationale for focusing on GP as a target for a preventative vaccine and providing leads for other clinical interventions.
REFERENCES
1. Alvarez, C. P., F. Lasala, J. Carrillo, A. L. Corbi, and R. Delgado. 2002. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 76:6841-6844. [PMC free article] [PubMed]
2. Baize, S., E. M. Leroy, M.-C. Georges-Courbot, M. Capron, J. Lansoud-Soukate, P. Debre, S. P. Fisher-Hoch, J. B. McCormick, and A. J. Georges. 1999. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat. Med. 5:423-426. [PubMed]
3. Baskerville, A., E. T. W. Bowen, G. S. Platt, L. B. McArdell, and D. I. H. Simpson. 1978. The pathology of experimental Ebola virus infection in monkeys. J. Pathol. 125:131-138. [PubMed]
4. Baskerville, A., S. P. Fisher-Hoch, G. H. Neild, and A. B. Dowsett. 1985. Ultrastructural pathology of experimental Ebola haemorrhagic fever virus infection. J. Pathol. 147:199-209. [PubMed]
5. Basler, C. F., X. Wang, E. Muhlberger, V. Volchkov, J. Paragas, H.-D. Klenk, A. Garcia-Sastre, and P. Palese. 2000. The Ebola virus VP35 protein functions as a type 1 IFN antagonist. Proc. Natl. Acad. Sci. USA 97:12289-12294. [PMC free article][PubMed]
6. Bowen, E. T., G. Lloyd, W. J. Harris, G. S. Platt, A. Baskerville, and E. E. Vella.1977. Viral haemorrhagic fever in southern Sudan and northern Zaire. Preliminary studies on the aetiological agent. Lancet i:571-573. [PubMed]
7. Bray, M., K. Davis, T. Geisbert, C. Schmaljohn, and J. Huggins. 1998. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. J. Infect. Dis. 178:651-661. [PubMed]
8. Chan, S. Y., C. J. Empig, F. J. Welte, R. F. Speck, A. Schmaljohn, J. F. Kreisberg, and M. A. Goldsmith. 2001. Folate receptor-α is a cofactor for cellular entry by Marburg and Ebola viruses. Cell 106:117-126. [PubMed]
9. Colebunders, R., and M. Borchert. 2000. Ebola haemorrhagic fever—a review. J. Infect. 40:16-20. [PubMed]
10. Connolly, B. M., K. E. Steele, K. J. Davis, T. W. Geisbert, W. M. Kell, N. K. Jaax, and P. B. Jahrling. 1999. Pathogenesis of experimental Ebola virus infection in guinea pigs. J. Infect. Dis. 179:S203-S217. [PubMed]
11. Donnelly, J. J., J. B. Ulmer, J. W. Shiver, and M. A. Liu. 1997. DNA vaccines.Annu. Rev. Immunol. 15:617-648. [PubMed]
12. Feldmann, H., H. Bugany, F. Mahner, H. D. Klenk, D. Drenckhahn, and H. J. Schnittler. 1996. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J. Virol. 70:2208-2214. [PMC free article] [PubMed]
13. Feldmann, H., S. T. Nichol, H. D. Klenk, C. J. Peters, and A. Sanchez. 1994. Characterization of filoviruses based on differences in structure and antigenicity of the virion glycoprotein. Virology 199:469-473. [PubMed]
14. Geisbert, T. W., P. Pushko, K. Anderson, J. Smith, K. J. Davis, and P. B. Jahrling. 2002. Evaluation in nonhuman primates of vaccines against Ebola virus.Emerg. Infect. Dis. 8:503-507. [PMC free article] [PubMed]
15. Gupta, M., S. Mahanty, M. Bray, R. Ahmed, and P. E. Rollin. 2001. Passive transfer of antibodies protects immunocompetent and immunodeficient mice against lethal Ebola virus infection without complete inhibition of viral replication. J. Virol. 75:4649-4654. [PMC free article] [PubMed]
16. Ito, H., S. Watanabe, A. Sanchez, M. A. Whitt, and Y. Kawaoka. 1999. Mutational analysis of the putative fusion domain of Ebola virus glycoprotein. J. Virol.73:8907-8912. [PMC free article] [PubMed]
17. Ito, H., S. Watanabe, A. Takada, and Y. Kawaoka. 2001. Ebola virus glycoprotein: proteolytic processing, acylation, cell tropism, and detection of neutralizing antibodies. J. Virol. 75:1576-1580. [PMC free article] [PubMed]
18. Jahrling, P. B., J. Geisbert, J. R. Swearengen, G. P. Jaax, T. Lewis, J. W. Huggins, J. J. Schmidt, J. W. LeDuc, and C. J. Peters. 1996. Passive immunization of Ebola virus-infected cynomolgus monkeys with immunoglobulin from hyperimmune horses. Arch. Virol. 11(Suppl.):135-140. [PubMed]
19. Jahrling, P. B., T. W. Geisbert, D. W. Dalgard, E. D. Johnson, T. G. Ksiazek, W. C. Hall, and C. J. Peters. 1990. Preliminary report: isolation of Ebola virus from monkeys imported to USA. Lancet 335:502-505. [PubMed]
20. Johnson, K. M., J. V. Lange, P. A. Webb, and F. A. Murphy. 1977. Isolation and partial characterisation of a new virus causing acute haemorrhagic fever in Zaire. Lanceti:569-571. [PubMed]
21. Kilby, J. M., S. Hopkins, T. M. Venetta, B. DiMassimo, G. A. Cloud, J. Y. Lee, L. Alldredge, E. Hunter, D. Lambert, D. Bolognesi, T. Matthews, M. R. Johnson, M. A. Nowak, G. M. Shaw, and M. S. Saag. 1998. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat. Med. 4:1302-1307. [PubMed]
22. Kindzelskii, A. L., Z. Yang, G. J. Nabel, R. F. Todd III, and H. R. Petty. 2000. Ebola virus secretory glycoprotein (sGP) diminishes FcγRIIIB-to-CR3 proximity on neutrophils. J. Immunol. 164:953-958. [PubMed]
23. Malashkevich, V. N., B. J. Schneider, M. L. McNally, M. A. Milhollen, J. X. Pang, and P. S. Kim. 1999. Core structure of the envelope glycoprotein GP2 from Ebola virus at 1.9-Å resolution. Proc. Natl. Acad. Sci. USA 96:2662-2667. [PMC free article][PubMed]
24. Maruyama, T., L. L. Rodriguez, P. B. Jahrling, A. Sanchez, A. S. Khan, S. T. Nichol, C. J. Peters, P. W. Parren, and D. R. Burton. 1999. Ebola virus can be effectively neutralized by antibody produced in natural human infection. J. Virol.73:6024-6030. [PMC free article] [PubMed]
25. Parren, P. W., T. W. Geisbert, T. Maruyama, P. B. Jahrling, and D. R. Burton.2002. Pre- and postexposure prophylaxis of Ebola virus infection in an animal model by passive transfer of a neutralizing human antibody. J. Virol. 76:6408-6412.[PMC free article] [PubMed]
26. Peters, C. J., and A. S. Khan. 1999. Filovirus diseases. Curr. Top. Microbiol. Immunol. 235:85-95. [PubMed]
27. Ploegh, H. L. 1998. Viral strategies of immune evasion. Science 280:248-253.[PubMed]
28. Sanchez, A., A. S. Khan, S. R. Zaki, G. J. Nabel, T. G. Ksiazek, and C. J. Peters.2001. Filoviridae: Marburg and Ebola viruses, p. 1279-1304. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott, Williams & Wilkins, Philadelphia, Pa.
29. Sanchez, A., S. G. Trappier, B. W. J. Mahy, C. J. Peters, and S. T. Nichol. 1996. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc. Natl. Acad. Sci. USA 93:3602-3607.[PMC free article] [PubMed]
30. Sanchez, A., Z. Yang, L. Xu, G. J. Nabel, T. Crews, and C. J. Peters. 1998. Biochemical analysis of the secreted and virion glycoproteins of Ebola virus. J. Virol.72:6442-6447. [PMC free article] [PubMed]
31. Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. Hill. 1998. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [PubMed]
32. Simmons, G., J. D. Reeves, C. C. Grogan, L. H. Vandenberghe, F. Baribaud, J. C. Whitbeck, E. Burke, M. J. Buchmeier, E. J. Soilleux, J. L. Riley, R. W. Doms, P. Bates, and S. Pohlmann. 2003. DC-SIGN and DC-SIGNR bind Ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology 305:115-123.[PubMed]
33. Simmons, G., R. J. Wool-Lewis, F. Baribaud, R. C. Netter, and P. Bates. 2002. Ebola virus glycoproteins induce global surface protein down-modulation and loss of cell adherence. J. Virol. 76:2518-2528. [PMC free article] [PubMed]
34. Ströher, U., E. West, H. Bugany, H.-D. Klenk, H.-J. Schnittler, and H. Feldmann. 2001. Infection and activation of monocytes by Marburg and Ebola viruses. J. Virol. 75:11025-11033. [PMC free article] [PubMed]
35. Sui, J., and W. A. Marasco. 2002. Evidence against Ebola virus sGP binding to human neutrophils by a specific receptor. Virology 303:9-14. [PubMed]
36. Sullivan, N. J., A. Sanchez, P. E. Rollin, Z.-Y. Yang, and G. J. Nabel. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature408:605-609. [PubMed]
36a. Sullivan, N. J., T. W. Geisbert, L. Xu, Z.-Y. Yang, M. Roederer, R. A. Koup, P. B. Jahrling, and G. J. Nabel. 2003. Accelerated vaccination for ebola virus hemorrhagic fever in non-human primates. Nature 424:681-684. [PubMed]
37. Takada, A., S. Watanabe, H. Ito, K. Okazaki, H. Kida, and Y. Kawaoka. 2000. Downregulation of β1 integrins by Ebola virus glycoprotein: implication for virus entry.Virology 278:20-26. [PubMed]
38. Tighe, H., M. Corr, M. Roman, and E. Raz. 1998. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol. Today 19:89-97. [PubMed]
39. Vanderzanden, L., M. Bray, D. Fuller, T. Roberts, D. Custer, and K. Spik.1998. DNA vaccines expressing either the GP or NP genes of Ebola virus protect mice from lethal challenge. Virology 246:134-144. [PubMed]
40. Villinger, F., P. E. Rollin, S. S. Brar, N. F. Chikkala, J. Winter, J. Sundstrom, S. R. Zaki, R. Swanepoel, A. Ansari, and C. J. Peters. 1999. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J. Infect. Dis. 179:S188-S191. [PubMed]
41. Volchkov, V. E., S. Becker, V. A. Volchkova, V. A. Ternovoj, A. N. Kotov, S. V. Netesov, and H. D. Klenk. 1995. GP mRNA of Ebola virus is edited by the Ebola virus polymerase and by T7 and vaccinia virus polymerases. Virology 214:421-430. [PubMed]
42. Volchkov, V. E., V. A. Volchkova, E. Muhlberger, L. V. Kolesnikova, M. Weik, O. Dolnik, and H.-D. Klenk. 2001. Recovery of infectious Ebola virus from complementary DNA: RNA editing of the GP gene and viral cytotoxicity. Science291:1965-1969. [PubMed]
43. Watanabe, S., A. Takada, T. Watanabe, H. Ito, H. Kida, and Y. Kawaoka. 2000. Functional importance of the coiled-coil of the Ebola virus glycoprotein. J. Virol.74:10194-10201. [PMC free article] [PubMed]
44. Weissenhorn, W., A. Carfi, K. H. Lee, J. J. Skehel, and D. C. Wiley. 1998. Crystal structure of the Ebola virus membrane fusion subunit, GP2, from the envelope glycoprotein ectodomain. Mol. Cell 2:605-616. [PubMed]
45. Wilson, J. A., M. Hevey, R. Bakken, S. Guest, M. Bray, A. L. Schmaljohn, and M. K. Hart. 2000. Epitopes involved in antibody-mediated protection from Ebola virus.Science 287:1664-1666. [PubMed]
46. Wyers, M., P. Formenty, Y. Cherel, L. Guigand, B. Fernandez, C. Boesch, and B. Le Guenno. 1999. Histopathological and immunohistochemical studies of lesions associated with Ebola virus in a naturally infected chimpanzee. J. Infect. Dis. 179:S54-S59. [PubMed]
47. Xu, L., A. Sanchez, Z. Yang, S. R. Zaki, E. G. Nabel, S. T. Nichol, and G. J. Nabel. 1998. Immunization for Ebola virus infection. Nat. Med. 4:37-42. [PubMed]
48. Yang, Z.-Y., H. J. Duckers, N. J. Sullivan, A. Sanchez, E. G. Nabel, and G. J. Nabel. 2000. Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat. Med. 6:886-889. [PubMed]
49. Yang, Z.-Y., R. Delgado, L. Xu, R. F. Todd, E. G. Nabel, A. Sanchez, and G. J. Nabel. 1998. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science 279:1034-1037. [PubMed]
50. Zaki, S. R., W. Shieh, P. W. Greer, C. S. Goldsmith, T. Ferebee, J. Katshitshi, F. Tshioko, M. Bwaka, R. Swanepoel, P. Calain, A. S. Khan, E. Lloyd, P. Rollin, T. G. Ksiazek, and C. J. Peters. 1999. A novel immunohistochemical assay for the detection of Ebola virus in skin: implications for diagnosis, spread, and surveillance of Ebola hemorrhagic fever. J. Infect. Dis. 179:S36-S47. [PubMed]
Thursday 12 February 2015
Thursday 12 February 2015
- 0 Comments
Folic AcidFolic acid is one of the B-complex vitamins required in order to produce red blood cells. Folic acid is a manufactured form of folate; folate can be found naturally in certain foods, such as leafy green vegetables, nuts, beans, and grains. Some cereals contain 100% of the daily value of folic acid a woman should take per day. If there is an insufficient amount of this vitamin, it can cause anemia. Because the body does not make much folic acid, it is useful to take a vitamin pill form to ensure that you get the recommended daily value.
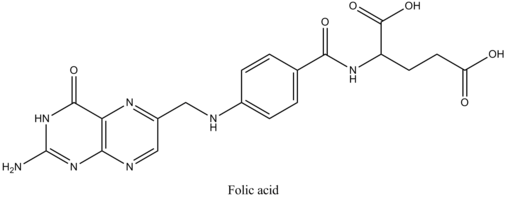
History
Many scientists around the 1920s thought that folate deficiency and anemia were the same condition. In the year 1931, a researcher named Lucy Wills led to determining folate as the nutrient needed to prevent anemia during pregnancy. In other words, folate was an important source needed during the state when a person is pregnant. Through this identification, Dr. Wills illustrated that anemia could be reversed with brewer's yeast. Thus, folate was seen as the corrective substance in brewer's yeast. In the year 1941, Mitchell and other people first separated and extracted folate from spinach leaves.
Furthermore, in 1943, Bob Stokstad who worked at the Lederle Laboratories of the American Cyanamid Company, isolated the pure crystalline form and was then able to determine folate chemical structure. Under the supervision and help of Director of Research Dr. Yellapragada Subbarao, a group called "folic acid boys" in the year 1945 was able to obtain folic acid in a pure crystalline form. This historical research project led to the synthesis of the antifolate aminopterin. Antifolate aminopterin is the first anticancer drug.
Then in the 1950s to the 1960s, many scientists started to study biochemical mechanisms and discovered the different actions for folate. This led to linking folate deficiency to neural tube defects. Overall, many US scientists noticed that food sold in markets contain really little folate; therefore, more food should contains folate to help people especailly those who are pregnant.
Foods Containing Folate
There are many healthy foods that are very high in folate. Some are listed below:
- Egg yolks
- Sunflower seeds
- Liver and Kidney Products
- Leafy vegetables such as turnip greens, lettuce, spinach
- Legumes such as beans, peas, and lentils
- Grain Products such pasta, cereal, bread
Food that contains few amount in folate:
- Fruits such as banana, raspberry, strawberry
- Juice such as orange or pineapple juice
Note that folate is naturally found in foods that are susceptible in high heat and UV light. Folate is also water soluble.
Folic Acid and Pregnancy
Folic acid has been proven to protect against birth defects during the first weeks of pregnancy. Such birth defects include spina bifida, which is the case involving the backbone and spinal canal not being able to fully close. It can also protect against anencephaly, a condition in which the brain does not develop. Babies born with anencephaly usually die before or shortly after they are born. [1] Women who are pregnant or are trying to get pregnant are recommended to take 400 to 800 micrograms of folic acid per day.
It is possible for folic acid to reduce chances of spinal or brain defects by nearly 70%. These diseases are known as neural tube defects which always occur when the spinal cord fails to close properly during development which is what spina bifida is.
Folic acid ultimately reduces the level of a potentially harmful compound called homocysteine. This is done by speeding up the conversion of homocysteine to methionine, a nontoxic amino acid that the human body prefers and needs. Scientists and researchers discovered that locking the enzyme MTHFR to its cofactor FAD allows folic acid to perform its unique function in the human body. Therefore, the Food and Drug Administration recommends all women of child-bearing age to supplement her diet with folic acid to prevent potential birth defects from occurring.
Side effects of taking folic acid include a skin rash, itchiness, redness, or difficulty breathing.
Folic acid can also be ingested through a balanced diet. This includes, fortified cereals, whole grains, fruits, veggies, beans, and other natural protein. Those looking to increase folic acid intake due to pregnancy can do it naturally through diet or both with supplements and food. It is important for males also to not neglect folic acid. Although they do not benefit from the birth defect preventions, folic acid is still a healthy supplement that is necessary in small doses.
Sperm Quality
It is commonly seen that folic acid can minimize the chromosomal defeats in sperm. Thus, folate is an important source for fertility in both men and women because it contributes to spermatogenesis. Basically, for both gender, it is crucial to receive a good amount of folate through the diet to avoid subfertility.
Heart Disease
Using folic acid will reduce homocysteine levels, but it will not minimize cardiovascular disease. However, this applies differently for women who are pregnant. Consuming folic acid during pregnancy may reduce the risk of heart defects in infants, which is a good thing. It may also reduce the risk for children to develop a syndrome called the metabolic syndrome.
Stroke
Even though taking folic acid does not reduce heart disease, it appears to reduce the risk of stroke. However, there are many reviews that indicate how only some individuals who take folic acid may in return minimize the risk of stroke. In other words, folic acid may only work on some people. It is being said that stroke reduction is consistent with the reduction in pulse pressure produced by folate supplementation of 5 mg per day. So for those who are likely to get heart disease, it is important to consume folate in every day diet. This is the reason why hyperhomocysteinemia or stroke patients are greatly encouraged by their doctors to take daily vitamin B which includes folic acid. Folic supplements are in expensive and are quite safe to use, but do not overdo it.
Cancer
Since many cancer cells tolerate folic acid and overexpress the folic acid receptor, this had led to the creation of anti-cancer drugs that target the folic acid receptor. There are investigations that proved that good levels of folic acid may be related to lower risk of stomach, esophageal, and ovarian cancers. However, the benefits of folic acid against cancer may only depend on when an individual is taking it and on the conditions of that particular person. This is because everyone tends to have a different immune system that reacts to certain things differently.
Moreover, for individuals who are already suffering from cancer or from precancerous condition may find that taking folic acid may not be helpful and can be damaging. Therefore, consuming a certain amount of folic acid is crucial for everyday diet, but it is important to note that excess of folate may promote tumor initiation. High folate intake promotes advanced carcinogenesis and low folate intake protects against early carcinogenesis. Hence, many doctors and public health recommend being super careful when taking folate and encourage not to intake too much folate.
Diets that are high in folate are related with the decreased risk of colorectal cancer. There are some researches that show how the association is stronger for folate that are taken from foods than folate from supplements. In relation to folate and one carbon metabolism, colorectal cancer is the most studied type of cancer. Furthermore, there are epidemiologic studies that suggest diets high in folate are associated with decreased risk of breast cancer. Studies also show that high dietary folate intake will minimize the risk of prostate cancer. Overall, there had been many studies dealing with folate acid to prevent many kinds of disease.
Subscribe to:
Posts (Atom)
