Friday, 19 December 2014
Integration and Excision of a Plasmid
Friday, 19 December 2014 by Unknown
Multicopy plasmids have greatly facilitated gene structure-function studies. However, the use of such plasmids can lead to high-copy-number artifacts, especially in physiological studies. Thus, several methods have been developed for recombining genes on bacterial chromosomes in order to study their functions in single copies. Such methods are frequently used to construct novel Escherichia coli strains that stably express foreign genes for use in both basic research and biotechnology (5, 18, 27). However, the development of strains encoding complex metabolic or regulatory pathways poses special problems that often require manipulating many genes and expressing them individually at different levels or under separate regulatory controls. To address these concerns, we have developed a series of plasmid-host systems for the introduction of multiple genes into the same cell in single copies. Our approach is based on genome targeting systems that utilize plasmids carrying a conditional-replication origin and a phage attachment (attP) site (17). We refer to our plasmids as CRIM (conditional-replication, integration, and modular) plasmids. CRIM plasmids can be integrated into or retrieved from their bacterial attachment (attB) site by supplying phage integrase (Int) without or with excisionase (Xis) in trans.
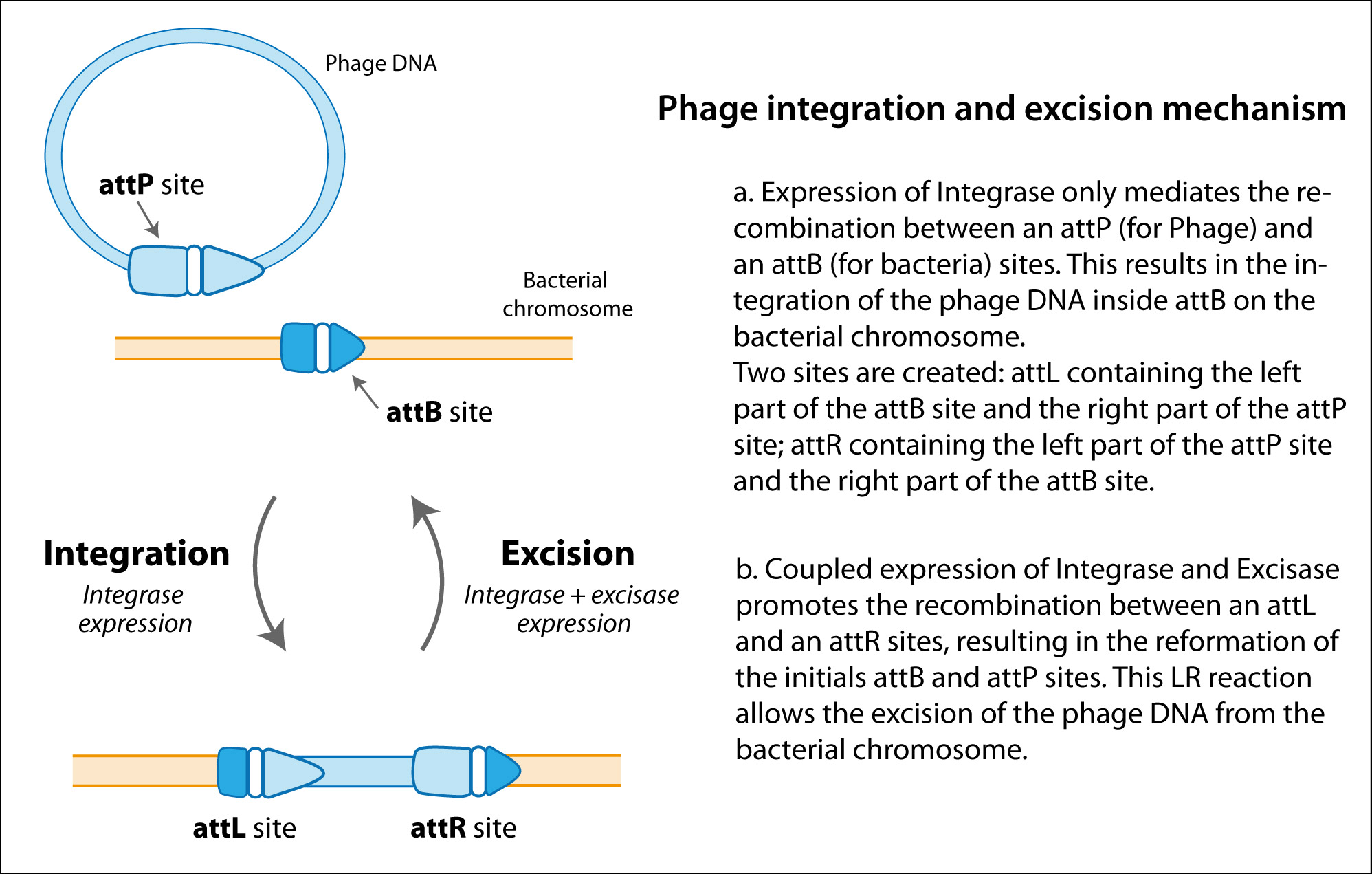
Advantages of our CRIM plasmid-host systems include the use of alternative attP andattB sites (for phages λ, HK022, ![[var phi]](http://www.ncbi.nlm.nih.gov/corehtml/pmc/pmcents/x03C6.gif) 80, P21, and P22) and different selectable markers (for chloramphenicol, gentamicin, kanamycin, spectinomycin and streptomycin, tetracycline, and trimethoprim resistance) in conjunction with a polylinker or promoter (ParaB, PrhaB,PrhaS, Ptac, Psyn1, and Psyn4) for ectopic expression of the cloned gene(s). These CRIM plasmids have the γ replication origin of R6K, which requires the trans-acting Π protein (encoded by pir) for replication. So, they replicate at a medium (15 per cell) or high (250 per cell) plasmid copy number in pir+ or pir-116 (high-copy-number mutant) E. coli hosts (28), respectively. Int helper plasmids are used for integration of CRIM plasmids into the corresponding chromosomal attB sites of normal (non-pir) hosts, which are nonpermissive for CRIM plasmid replication. Xis/Int helper plasmids are used for excision (“curing”) of the respective CRIM plasmids from the chromosome, e.g., to verify that phenotypes are due to their presence. Xis/Int helper plasmids are also used for retrieval (cloning) of CRIM plasmids from the chromosome, e.g., to recover a particular CRIM plasmid after screening of CRIM plasmid or mutant libraries.
80, P21, and P22) and different selectable markers (for chloramphenicol, gentamicin, kanamycin, spectinomycin and streptomycin, tetracycline, and trimethoprim resistance) in conjunction with a polylinker or promoter (ParaB, PrhaB,PrhaS, Ptac, Psyn1, and Psyn4) for ectopic expression of the cloned gene(s). These CRIM plasmids have the γ replication origin of R6K, which requires the trans-acting Π protein (encoded by pir) for replication. So, they replicate at a medium (15 per cell) or high (250 per cell) plasmid copy number in pir+ or pir-116 (high-copy-number mutant) E. coli hosts (28), respectively. Int helper plasmids are used for integration of CRIM plasmids into the corresponding chromosomal attB sites of normal (non-pir) hosts, which are nonpermissive for CRIM plasmid replication. Xis/Int helper plasmids are used for excision (“curing”) of the respective CRIM plasmids from the chromosome, e.g., to verify that phenotypes are due to their presence. Xis/Int helper plasmids are also used for retrieval (cloning) of CRIM plasmids from the chromosome, e.g., to recover a particular CRIM plasmid after screening of CRIM plasmid or mutant libraries.
![[var phi]](http://www.ncbi.nlm.nih.gov/corehtml/pmc/pmcents/x03C6.gif) 80, P21, and P22) and different selectable markers (for chloramphenicol, gentamicin, kanamycin, spectinomycin and streptomycin, tetracycline, and trimethoprim resistance) in conjunction with a polylinker or promoter (ParaB, PrhaB,PrhaS, Ptac, Psyn1, and Psyn4) for ectopic expression of the cloned gene(s). These CRIM plasmids have the γ replication origin of R6K, which requires the trans-acting Π protein (encoded by pir) for replication. So, they replicate at a medium (15 per cell) or high (250 per cell) plasmid copy number in pir+ or pir-116 (high-copy-number mutant) E. coli hosts (28), respectively. Int helper plasmids are used for integration of CRIM plasmids into the corresponding chromosomal attB sites of normal (non-pir) hosts, which are nonpermissive for CRIM plasmid replication. Xis/Int helper plasmids are used for excision (“curing”) of the respective CRIM plasmids from the chromosome, e.g., to verify that phenotypes are due to their presence. Xis/Int helper plasmids are also used for retrieval (cloning) of CRIM plasmids from the chromosome, e.g., to recover a particular CRIM plasmid after screening of CRIM plasmid or mutant libraries.
80, P21, and P22) and different selectable markers (for chloramphenicol, gentamicin, kanamycin, spectinomycin and streptomycin, tetracycline, and trimethoprim resistance) in conjunction with a polylinker or promoter (ParaB, PrhaB,PrhaS, Ptac, Psyn1, and Psyn4) for ectopic expression of the cloned gene(s). These CRIM plasmids have the γ replication origin of R6K, which requires the trans-acting Π protein (encoded by pir) for replication. So, they replicate at a medium (15 per cell) or high (250 per cell) plasmid copy number in pir+ or pir-116 (high-copy-number mutant) E. coli hosts (28), respectively. Int helper plasmids are used for integration of CRIM plasmids into the corresponding chromosomal attB sites of normal (non-pir) hosts, which are nonpermissive for CRIM plasmid replication. Xis/Int helper plasmids are used for excision (“curing”) of the respective CRIM plasmids from the chromosome, e.g., to verify that phenotypes are due to their presence. Xis/Int helper plasmids are also used for retrieval (cloning) of CRIM plasmids from the chromosome, e.g., to recover a particular CRIM plasmid after screening of CRIM plasmid or mutant libraries.Since integration and retrieval involve phage-site-specific recombination events, the original and recovered plasmids are identical. CRIM plasmids can therefore be used for the construction of gene (or mutant) libraries that can be directly integrated into bacterial chromosomes in single copies for screening or selection purposes. Afterwards, CRIM plasmids can be retrieved from individual cells or en masse. The recovered plasmids can then be propagated as plasmids for molecular analysis or integrated directly into the chromosomes of other hosts for subsequent processing without further in vitro manipulation steps. We previously found similar oriRγ attλ plasmids to be extremely useful in mutagenesis studies, especially when it was important that the mutated gene be free of plasmid copy number effects (16). We also found them to be useful in studying genes from diverse bacteria, including gram-negative and -positive cells (14, 15, 25, 34). Our versatile CRIM plasmid-host systems should be widely useful in gene structure-function studies. Here we describe our basic set of CRIM plasmids, the requisite helper plasmids, and how to use them.
Subscribe to:
Post Comments (Atom)


0 Responses to “Integration and Excision of a Plasmid”
Post a Comment